SARAH L. EDMONDS,1* JAMES MANN,2 ROBERT R. MCCORMACK,3 DAVID R. MACINGA,1 CHRISTOPHER M. FRICKER,1 JAMES W. ARBOGAST,1 AND MICHAEL J. DOLAN1
1GOJO Industries, Inc., P.O. Box 991, Akron, Ohio 44309; 2Handwashing for Life, 1216 Flamingo Parkway, Libertyville, Illinois 60048; and 3BioScience Laboratories, Inc., 300 North Willson Avenue, Suite 1, Bozeman, Montana 59715, USA
MS 10-220: Received 25 May 2010/Accepted 27 August 2010
ABSTRACT
The risk of inadequate hand hygiene in food handling settings is exacerbated when water is limited or unavailable, thereby making washing with soap and water difficult. The SaniTwice method involves application of excess alcohol-based hand sanitizer (ABHS), hand ‘‘washing’’ for 15 s, and thorough cleaning with paper towels while hands are still wet, followed by a standard application of ABHS. This study investigated the effectiveness of the SaniTwice methodology as an alternative to hand washing for cleaning and removal of microorganisms. On hands moderately soiled with beef broth containing Escherichia coli (ATCC 11229), washing with a nonantimicrobial hand washing product achieved a 2.86 (¡0.64)-log reduction in microbial contamination compared with the baseline, whereas the SaniTwice method with 62% ethanol (EtOH) gel, 62% EtOH foam, and 70% EtOH advanced formula gel achieved reductions of 2.64 ¡ 0.89, 3.64 ¡ 0.57, and 4.61 ¡ 0.33 log units, respectively. When hands were heavily soiled from handling raw hamburger containing E. coli, washing with nonantimicrobial hand washing product and antimicrobial hand washing product achieved reductions of 2.65 ¡ 0.33 and 2.69 ¡ 0.32 log units, respectively, whereas SaniTwice with 62% EtOH foam, 70% EtOH gel, and 70% EtOH advanced formula gel achieved reductions of 2.87 ¡ 0.42, 2.99 ¡ 0.51, and 3.92 ¡ 0.65 log units, respectively. These results clearly demonstrate that the in vivo antibacterial efficacy of the SaniTwice regimen with various ABHS is equivalent to or exceeds that of the standard hand washing approach as specified in the U.S. Food and Drug Administration Food Code. Implementation of the SaniTwice regimen in food handling settings with limited water availability should significantly reduce the risk of foodborne infections resulting from inadequate hand hygiene.
Foodborne diseases are a serious public health concern (3, 4, 15), but despite preventive efforts there has been little recent progress in reducing infections caused by foodborne pathogens (6). Faulty food handling practices, particularly improper hand washing, contribute significantly to the risk for foodborne disease (11–13, 19, 25–27, 29). Proper hand hygiene reduces the risk of transmission of pathogens from hands to food (7, 20, 21) and is associated with a reduction in gastrointestinal illness (2, 8, 18). The U.S. Food and Drug Administration (FDA) Food Code for retail establishments requires hand washing as a preventive method and provides specific guidance on proper hand washing procedures (30). The five-step hand washing procedure outlined in the FDA Food Code consists of (i) rinsing under warm running water, (ii) applying the manufacturer-recommended amount of cleaning compound, (iii) rubbing the hands vigorously, (iv) rinsing thoroughly under warm running water, and (v) thoroughly drying the hands with individual paper towels, a continuous clean towel system, or a heated or pressurized hand air drying device. According to the Food Code, alcohol-based hand sanitizers (ABHS) may be used in retail and food service only after proper hand washing.
ABHS are recommended as an alternative to traditional hand washing in the health care setting (5). Alcohols are highly effective against a range of bacterial pathogens, fungi, enveloped viruses, and certain nonenveloped viruses (2, 10). Although considered to be ineffective antimicrobial agents in the presence of visible dirt or proteinaceous material, alcohol- containing products were more effective than those containing triclosan (2, 14) or detergents (17) for removing microorgan- isms from hands contaminated with organic material. In health care facilities and other environments, easily accessible ABHS have resulted in greater hand hygiene compliance and reduction in infections (1, 9, 16, 31). Although ABHS are approved for use in the health care environment, the FDA does not regard these agents as adequate substitutes for soap and water in the food service setting (30).
A reliable hand hygiene method is needed for food service settings in which adequate hand washing facilities are limited or unavailable. These settings include portable bars, buffet lines, outdoor events, and catering functions at which the only available hand hygiene facility often is either ‘‘trickle hand washing’’ (i.e., hand washing done from a portable container of water over a bucket or other type of basin) or simply the use of a paper towel or damp cloth to rub the hands. These methods may be inadequate for proper hand cleansing.
SaniTwice (a registered trademark with James Mann, Handwashing for Life, Libertyville, IL) is a two-stage hand cleansing protocol that is performed using ABHS when water is not available. In this study, we evaluated the microbiological efficacy of the SaniTwice method on the hands of adult human participants. These studies were designed to assess (i) the antimicrobial efficacy of various ABHS used with the SaniTwice regimen as compared with that of a standard hand washing method with soap and water on soiled hands and (ii) the impact of the active ingredient and/or formulation of a hand sanitizer on antibacterial efficacy when used in a SaniTwice regimen.
MATERIALS AND METHODS
Test products. All test products in this study were manufactured by GOJO Industries (Akron, OH). Two hand washing products were evaluated: a nonantimicrobial product (GOJO Luxury Foam Handwash) and an antimicrobial product (MICRELL Antibacterial Foam Handwash, 0.5% chloroxylenol active). Four ABHS also were evaluated: a 62% ethanol (EtOH) gel (PURELL Instant Hand Sanitizer Food Code Compliant), a 62% EtOH foam (PURELL Instant Hand Sanitizer Foam), a 70% EtOH gel (PURELL 70 Instant Hand Sanitizer), and a 70% EtOH Advanced Formula (AF) gel (PURELL Instant Hand Sanitizer Advanced Formula VF481).
Overall study design. Three studies were conducted by BioScience Laboratories (Bozeman, MT) to determine the in vivo antimicrobial efficacy of various test product configurations under conditions of moderate or heavy soil. The order of use of each product was determined randomly. A two-step testing sequence was used for all products. Each volunteer completed the baseline cycle, where hands were contaminated with moderate or heavy soil (as described below) containing Escherichia coli (ATCC 11229), and samples were collected for baseline bacterial counts. Following the baseline sampling, participants completed a 30-s nonmedicated soap wash followed by the product evaluation cycle, which consisted of a contamination procedure, application of the test product, and subsequent hand sampling. Between uses of different test products, participants decontaminated their hands with a 1-min 70% EtOH rinse, air drying, and a 30-s nonmedicated soap wash. A minimum of 20 min elapsed before the next testing sequence began. Baseline and postapplication samples were evaluated for the presence of E. coli. Testing was performed according to the FDA health care personnel hand washing product evaluation method (28) and modified as described previously (22).
The study was approved by the Gallatin Institutional Review, an independent review board unaffiliated with BioScience Laboratories, and was conducted in compliance with Good Clinical Practice and Good Laboratory Practice regulations. All participants provided written informed consent.
Participants. The study enrolled healthy adults with two hands. All participants were free of dermal allergies or skin disorders on the hands or forearms.
Preparation of inoculum. E. coli was used to test the efficacy of the test procedures. A 2-liter flask was filled with
1,000 ml of tryptic soy broth: 30.0 g of dehydrated tryptic soy broth medium (BD, Franklin Lakes, NJ) added to 1 liter of deionized water, heated, and sterilized for a final pH of 7.3 ¡ 0.20. The broth was inoculated with 1.0 ml of a 24-h culture of E. coli grown from a cryogenic stock culture. The flask was incubated for 24 h, and the suspension was used for challenge.
Hand contamination procedures. For the moderate soil study, a 24-h culture of E. coli was suspended in beef broth (Swanson low sodium beef broth, Campbell Soup Company, Camden, NJ) at 1 | 109 CFU/ml. Three aliquots of 1.5 ml were transferred into each participant’s cupped hands. Each aliquot was distributed over the entire front and back surfaces of the hands up to the wrists during a 20-s period and allowed to air dry for 30 s after the first and second aliquots and for 90 s after the third aliquot. After samples were collected for baseline bacterial counts and hands were decontaminated with a 30-s wash with non- medicated soap, a second cycle of contamination was initiated. After the 90-s final drying step, participants applied the randomly assigned test product.
For the heavy soil study, 5.0-ml aliquots of the challenge suspension of E. coli were transferred to 4-oz (113-g) portions of sterile 90% lean ground beef and distributed evenly with gloved hands to achieve contamination levels of approximately 5.0 | 108 CFU per portion. Each participant then kneaded the inoculated raw hamburger for 2 min. Hands were air dried for 90 s and then sampled for baseline counts. After a 30-s decontamination with nonmedicated soap, the cycle was repeated, and the test product was applied.
Test article or product application and SaniTwice procedure. The hand washing procedure used for the nonantimi- crobial and antimicrobial hand washing products was consistent with Food Code specifications. Table 1 shows the stepwise product application procedures for all test configurations.
Bacterial recovery and microbial enumeration. Within 1 min after contamination for baseline evaluation or after product application, powder-free sterile latex gloves were placed on each participant’s hands and secured above the wrist, and 75 ml of sterile stripping fluid (0.4 g of KH2PO4, 10.1 g of Na2HPO4, and 1.0 g of isooctylphenoxypolyethoxyethanol in 1 liter of distilled water, pH adjusted to 7.8) was transferred into each glove. Following a 60-s massage of the hands through the gloves, a 5.0-ml aliquot of the glove rinsate sample was removed and diluted in 5.0 ml of Butterfield’s phosphate buffer solution with product neutralizers. Each aliquot was serially diluted in neutralizing solution, and appropriate dilutions were plated in duplicate onto MacConkey agar plates (BD; 50.0 g of dehydrated medium added to 1 liter of deionized water, heated, and sterilized; final pH, 7.1 ¡ 0.2) and incubated for 24 to 48 h at 30uC. Colonies were counted and data were recorded using the computerized Q-COUNT plate-counting systems (Advanced Instruments, Inc., Norwood, MA).
Data analysis and statistical considerations. The estimated log transformed number of viable microorganisms recovered from each hand (the R value) was determined using the formula R ~ log(75 | Ci | 10D | 2), where 75 is the amount (in milliliters) of stripping solution instilled into each glove, Ci is the arithmetic average colony count of the two plate counts at a particular dilution, D is the dilution factor, and 2 is the neutralization dilution.
Descriptive statistics and confidence intervals were calculated using the 0.05 level of significance for type I (alpha) error. Statistical calculations of means and standard deviations were generated for the log recovery data from baseline samples, post product application samples, and the log differences between baseline and post application samples. Product comparisons were made using a one-way analysis of variance with post hoc analysis (Bonferroni’s multiple comparison test) using the 0.05 level of significance for alpha error.
TABLE 1. Test product application proceduresa
| Step | Food Code-compliant procedure for hand washing products | SaniTwiceb procedure for ABHS | Procedure for 70% AF gel |
|---|---|---|---|
| 1 | Wet hands with water at 40°C | Dispense ~3 ml of product into cupped hands | Dispense ~1.5 ml of product into cupped hands |
| 2 | Apply ~1.5 ml of product | Rub vigorously over hands for 15 s to simulate washing | Rub hands together until dry |
| 3 | Lather for 15 s | Clean thoroughly with two paper towels | |
| 4 | Rinse with water for 10 s | Dispense additional ~1.5 ml of product | |
| 5 | Pat dry with two paper towels | Rub hands together until dry |
a All application procedures were initiated within 10 s of completing the 90-s drying step.
b SaniTwice is a registered trademark with James Mann (Handwashing for Life, Libertyville, IL).
RESULTS
Reduction in microbial contamination of moderately soiled hands. Two studies were conducted to evaluate microbial count reductions on hands that had been contaminated by handling beef broth containing E. coli. Reductions from baseline produced by the five test product configurations in these two studies are shown in Figure 1.
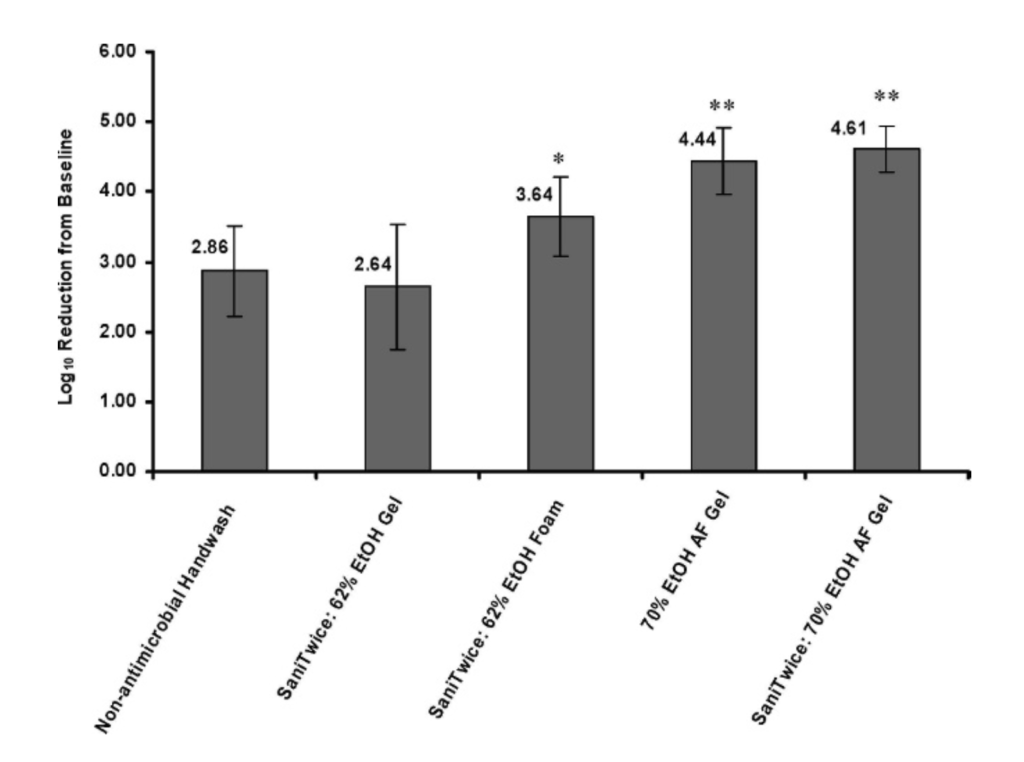
FIGURE 1. Log reduction from baseline for microbial contamination of hands moderately soiled with contaminated beef broth after application of test products. Error bars represent standard deviation. Data are from two separate studies. In study 1 (n ~ 11), nonantimicrobial hand washing product and SaniTwice with 62% EtOH gel were compared. In study 2 (n ~ 12), the conditions evaluated were nonantimicrobial hand washing product, Sani- Twice with 62% EtOH foam, 70% EtOH AF gel without SaniTwice, and SaniTwice with 70% EtOH AF gel. Results for nonantimicrobial hand washing product represent pooled data from both studies. * P , 0.05 for SaniTwice with 62% EtOH foam versus nonantimicrobial hand washing product or SaniTwice with 62% EtOH gel. ** P , 0.05 for 70% EtOH AF gel or for SaniTwice with 70% AF gel versus nonantimicrobial hand washing product, SaniTwice with 62% EtOH gel, or SaniTwice with 62% EtOH foam.
All SaniTwice regimens were equivalent to or better than the Food Code hand washing protocol. Reductions from baseline ranged from 2.64 ¡ 0.89 log CFU/ml for SaniTwice with the 62% EtOH gel to 4.61 ¡ 0.33 log CFU/ml for SaniTwice with the 70% EtOH AF gel.
SaniTwice using the 62% EtOH gel was equivalent to the nonantimicrobial Food Code hand washing protocol. However, SaniTwice using the 62% EtOH foam (3.64 ¡ 0.57-log reduction) was more effective than SaniTwice with the 62% EtOH gel and the Food Code hand washing protocol (P , 0.05).
The 70% EtOH AF gel was the most effective sanitizing product. When used independently, it was significantly more effective (4.44 ¡ 0.47-log reduction) than SaniTwice with 62% EtOH foam or 62% EtOH gel or the nonantimicrobial hand washing product (P , 0.05 for all comparisons). Although the log reduction data suggest that SaniTwice with 70% EtOH AF gel (4.61 ¡ 0.33-log reduction) was equivalent to the 70% EtOH AF gel used independently, this lack of differentiation was most likely due to the limitations of the assay. The 4.61-log reduction was at the limit of detection for all participants using 70% EtOH AF gel with SaniTwice but for only half the participants using 70% EtOH AF gel alone. Therefore, the log reductions produced by the 70% EtOH AF gel after either a single sanitization or the SaniTwice regimen are likely underestimated, and the log reductions in both cases would likely be higher if the limits of detection were lower.
Reduction in microbial contamination of heavily soiled hands. Figure 2 shows microbial count reductions produced by test product configurations on hands that had been contaminated by handling ground beef containing E. coli. All SaniTwice regimens tested were equivalent to or better than the Food Code hand washing protocol, indicating that under conditions of heavy soil, the SaniTwice procedure is as effective as hand washing. The performance of the antimicrobial hand washing product was equivalent to that of the nonantimicrobial hand washing product in this heavy soil challenge, with log reductions of 2.69 ¡ 0.32 and 2.65 ¡ 0.33, respectively. SaniTwice with the 70% EtOH AF gel outperformed all other sanitizer configurations tested and was superior to hand washing for reduction of organisms on heavily soiled hands (P , 0.05 for comparisons of SaniTwice with 70% EtOH AF gel versus each of the other procedures).
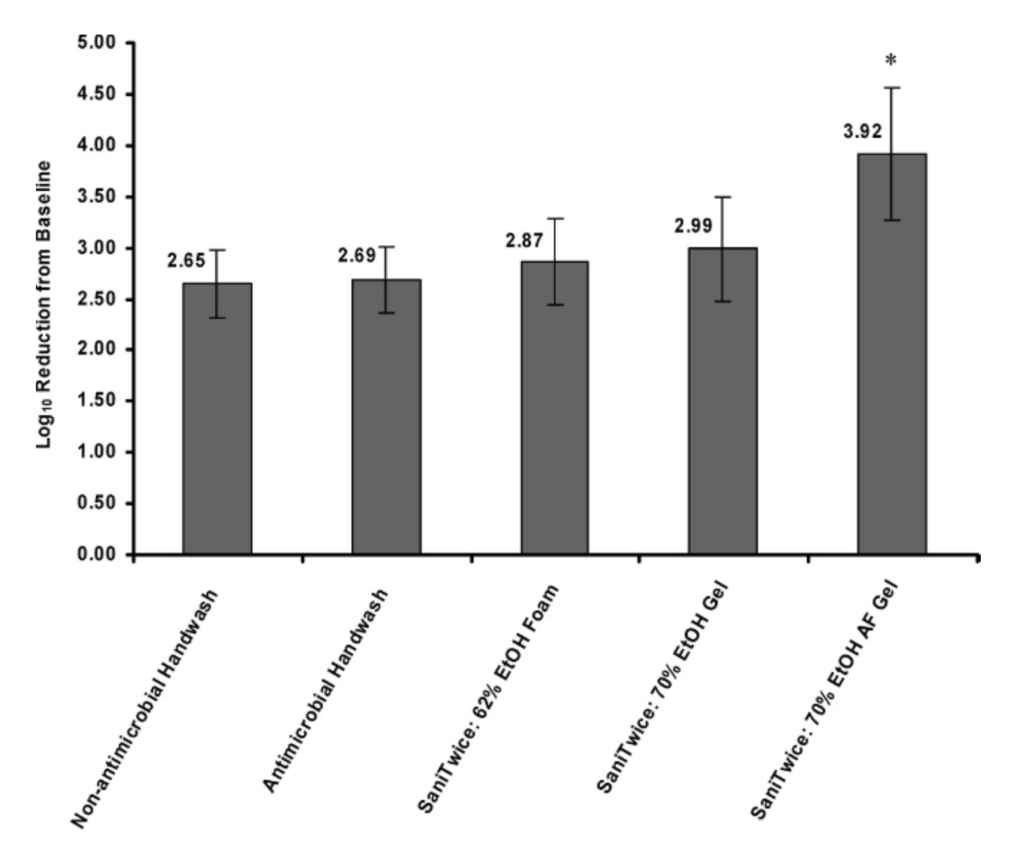
FIGURE 2. Log reduction from baseline for microbial contam- ination of hands heavily soiled with contaminated uncooked hamburger after application of test products and protocols. Error bars represent standard deviation. Data are from study 3 (n ~ 15), in which five test configurations were evaluated. * P , 0.05 for SaniTwice with 70% AF gel versus nonantimicrobial hand washing product, antimicrobial hand washing product, SaniTwice with 62% EtOH foam, or SaniTwice with 70% EtOH gel.
Two ABHS used with SaniTwice under both moderate and heavy soil conditions produced greater log reductions in the moderate soil condition. Mean log reductions using SaniTwice (moderate versus heavy soil) were 3.64 versus 2.87 for 62% EtOH foam and 4.61 versus 3.92 for 70% EtOH AF gel.
DISCUSSION
The SaniTwice method for hand disinfection was equivalent or superior to hand washing with soap and water for reducing viable bacteria on hands in the presence of representative food soils. Although the raw hamburger was a more difficult soil to penetrate, as demonstrated by approximately 1.0-log lower reductions compared with challenge by contaminated beef broth, the SaniTwice method with ABHS was equivalent to hand washing even under this worst-case simulation, underscoring the efficacy of this new method and indicating a potentially greater margin of safety.
The ABHS products used in this study exhibited a range of antimicrobial efficacy, suggesting that product formulation and the concentration of active ingredient may play a role in the observed efficacy. The impact of formulation was indicated by the significantly higher efficacy of the 62% EtOH foam compared with the 62% EtOH gel when challenged with moderate soil. This difference may be due to the additional foaming surfactants in the foam formulation, which may aid in lifting and removing bacteria and soil from the hands during the SaniTwice procedure. In addition, SaniTwice with the 70% EtOH AF gel was superior to SaniTwice with the 70% EtOH gel and 62% EtOH foam under heavy soil conditions. The 70% EtOH AF gel, whether tested as a single
application or with the SaniTwice method, was superior to hand washing and to the 62% EtOH gel or foam under moderate soil conditions. The 4.44-log reduction with a single use of the 70% EtOH AF gel demonstrates its high antimicrobial efficacy, which is further enhanced when used with the SaniTwice method. The 70% EtOH AF gel contains a patent-pending blend of ingredients that enhance the activity of the alcohol and likely contribute to the high efficacy observed in this study. The SaniTwice procedure gives the benefit of skin cleansing and soil removal, which is not obtained with single use of a product. The efficacy of ABHS used with SaniTwice against nonenveloped enteric viruses, which are more difficult to eradicate, remains to be determined.
In support of previous findings (23), the findings in this study indicate that the decontamination efficacy was similar for the antimicrobial and nonantimicrobial hand washing products under heavy soil conditions, suggesting that the cleansing properties of the surfactants in these soaps and the mechanical action of hand washing may be the primary contributors to efficacy rather than the antimicrobial activity of any constituent of the formulations. It is expected that with heavy hand soiling, the surfactant effect drives efficacy, and typical antibacterial constituents will have little additional effect.
In this study, SaniTwice was an effective hand hygiene regimen at least equivalent to hand washing with soap and water for reducing microbial contamination, even under worst case conditions of high bacterial load and heavy food soils. The current FDA Food Code allows use of ABHS only on hands that have been cleaned according to the recommended hand washing protocol (30). However, other than substitution of an ABHS for soap and water, the SaniTwice protocol mirrors the FDA-specified hand wash- ing sequence. SaniTwice is at least as effective as hand washing when used with standard-efficacy ABHS; when used with a high-efficacy ABHS, the SaniTwice protocol is superior to washing with soap and water. The Food Code provides few specific recommendations for achieving good hand hygiene when water (or other hand washing supplies and equipment) is unavailable or limited. The Food Code (Section 2-301.16) severely restricts hand sanitizers by allowing use only after proper hand washing or in situations in which no direct contact with food occurs (30).
A potential solution to this gap in food safety practices is SaniTwice. The SaniTwice studies described here provide convincing scientific rationale for including the SaniTwice approach in the Food Code as an alternative method of hand hygiene when standard hand washing is impractical. The simplicity and ease of use of the SaniTwice method, which requires only a supply of ABHS and paper towels, should allow this protocol to be applied to various food service settings and other areas in which hand hygiene is needed but safe water is unavailable or in short supply.
The findings in the present study support and extend those from previous studies; ABHS used alone or in combination with hand washing can be effective for decontaminating hands in the presence of organic soils (17, 23, 24). A well-formulated ABHS in conjunction with the SaniTwice regimen can have high efficacy, even in the presence of high organic load. Therefore, a reevaluation of the longstanding paradigm defining the use of ABHS in the presence of organic soils in both food handling and health care environments is warranted.
ACKNOWLEDGMENTS
Lakshmi Kamath and Meher Dustoor assisted in the preparation of this manuscript for publication.
* Author for correspondence. Tel: 330-255-6745; Fax: 330-255-6083: E-mail: edmondss@gojo.com.
REFERENCES
- Bischoff, W. E., T. M. Reynolds, C. N. Sessler, M. B. Edmond, and R. P. Wenzel. 2000. Handwashing compliance by health care workers: the impact of introducing an accessible, alcohol-based hand antiseptic. Arch. Intern. Med. 160:1017–1021.
- Bloomfield, S., A. Aiello, B. Cookson, C. O’Boyle, and E. Larson. 2007. The effectiveness of hand hygiene procedures in reducing the risks of infections in home and community settings including handwashing and alcohol-based hand sanitizers. Am. J. Infect. Control 35:S27–S64.
- Buzby, J. C., and T. Roberts. 1997. The economics of enteric infections: human foodborne disease costs. Gastroenterology 136: 1851–1862.
- Buzby, J. C., and T. Roberts. 2009. Economic costs and trade impacts of microbial foodborne illness. World Health Stat. Q. 50:57–66.
- Centers for Disease Control and Prevention. 2002. Guidelines for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Morb. Mortal. Wkly. Rep. 51:1–56.
- Centers for Disease Control and Prevention. 2009. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states. 2008. Morb. Mortal. Wkly. Rep. 58:333–337.
- Courtenay, M., L. Ramirez, B. Cox, I. Han, X. Jiang, and P. Dawson. 2005. Effects of various hand hygiene regimes in removal and/or destruction of Escherichia coli on hands. Food Serv. Technol. 5:77– 84.
- Curtis, V., and S. Cairncross. 2003. Effect of washing hands with soap on diarrhea risk in the community: a systematic review. Lancet Infect. Dis. 3:275–281.
- Fendler, E. J., Y. Ali, B. S. Hammond, M. K. Lyons, M. B. Kelley, and N. A. Vowell. 2002. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am. J. Infect. Control 30: 226–233.
- Fendler, E. J., and P. Groziak. 2002. Efficacy of alcohol-based hand sanitizers against fungi and viruses. Infect. Control Hosp. Epidemiol. 23:61–62.
- Green, L. R., V. Radke, R. Mason, L. Bushnell, D. W. Reimann, J. C. Mack, M. D. Motsinger, T. Stigger, and C. A. Selman. 2007. Factors related to food worker hand hygiene practices. J. Food Prot. 70:661– 666.
- Green, L. R., C. A. Selman, V. Radke, D. Ripley, J. C. Mack, D. W. Reimann, T. Stigger, M. Motsinger, and L. Bushnell. 2006. Food worker hand washing practices: an observation study. J. Food Prot. 69:2417–2423.
- Guzevich, J., and M. Ross. 1999. Evaluation of risks related to microbiological contamination of ready-to-eat food by food prepa- ration workers and the effectiveness of interventions to minimize those risks. Available at: http://www.fda.gov/Food/FoodSafety/ RetailFoodProtection/ucm210138.htm. Accessed 25 March 2010.
- Haas, C. N., J. R. Marie, J. B. Rose, and C. P. Gerba. 2005. Assessment of benefits from use of antimicrobial hand products: reduction in risk from handling ground beef. Int. J. Hyg. Environ. Health 208:461–466.
- Hoffmann, S., P. Fischbeck, A. Krupnick, and M. McWilliams. 2007. Using expert elicitation to link foodborne illnesses in the United States to foods. J. Food Prot. 70:1220–1229.
- Hugonnet, S., T. V. Perneger, and D. Pittet. 2002. Alcohol-based handrub improves compliance with hand hygiene in intensive care units. Arch. Intern. Med. 162:1037–1043.
- Larson, E., and L. Bobo. 1992. Effective hand degerming in the presence of blood. J. Emerg. Med. 10:7–11.
- Luby, S. P., M. Agboatwalla, J. Painter, A. Altaf, W. L. Billhimer, and R. M. Hoekstra. 2004. Effect of intensive handwashing promotion on childhood diarrhea in high-risk communities in Pakistan: a randomized controlled trial. JAMA (J. Am. Med. Assoc.) 291:2547–2554.
- Lues, J., and I. Van Tonder. 2007. The occurrence of indicator bacteria on hands and aprons of food handlers in the delicatessen sections of a retail group. Food Control 18:326–332.
- Michaels, B., C. Keller, M. Blevins, G. Paoli, T. Ruthman, E. Todd, and C. Griffith. 2004. Prevention of food worker transmission of foodborne pathogens: risk assessment and evaluation of effective hygiene intervention strategies. Food Serv. Technol. 4:31–49.
- Montville, R., Y. Chen, and D. W. Schaffner. 2002. Risk assessment of hand washing efficacy using literature and experimental data. Int. J. Food Microbiol. 73:305–313.
- Paulson, D. 1999. A suggested method for evaluating foodhandler/ processor handwash formulations. Dairy Food Environ. Sanit. 19: 546–550.
- Paulson, D., C. Riccardi, C. Beausoleil, E. Fendler, M. Dolan, L. Dunkerton, and R. Williams. 1999. Efficacy evaluation of four hand cleansing regimens for food handlers. Dairy Food Environ. Sanit. 19: 680–684.
- Schaffner, D. W., and K. M. Schaffner. 2007. Management of risk of microbial cross-contamination from uncooked frozen hamburgers by alcohol-based hand sanitizer. J. Food Prot. 70:109–113.
- Todd, E. C. D., J. D. Greig, C. A. Bartleson, and B. S. Michaels. 2007. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 2. Description of outbreaks by size, severity, and settings. J. Food Prot. 70:1975–1993.
- Todd, E. C. D., J. D. Greig, C. A. Bartleson, and B. S. Michaels. 2007. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 3. Factors contributing to outbreaks and description of outbreak categories. J. Food Prot. 70:2199–2217.
- Todd, E. C. D., J. D. Greig, C. A. Bartleson, and B. S. Michaels. 2008. Outbreaks where food workers have been implicated in the spread of foodborne disease. Part 5. Sources of contamination and pathogen excretion from infected persons. J. Food Prot 71:2582– 2595.
- U.S. Food and Drug Administration. 1994. Tentative final mono- graph for health-care antiseptic drug products: proposed rule. Fed. Regist. 59:31441–31452.
- U.S. Food and Drug Administration. 2004. FDA report on the occurrence of foodborne illness risk factors in selected institutional foodservice, restaurant, and retail food store facility types. Available at: http://www.fda.gov/Food/FoodSafety/RetailFoodProtection/ FoodborneIllnessandRiskFactorReduction/RetailFoodRiskFactor Studies/ucm089696.htm#execsum. Accessed 25 March 2010.
- U.S. Food and Drug Administration. 2009. Food Code 2009. Avail- able at: http://www.fda.gov/Food/FoodSafety/RetailFoodProtection/ FoodCode/FoodCode2009/. Accessed 25 March 2010.
- White, C., R. Kolble, R. Carlson, N. Lipson, M. Dolan, Y. Ali, and M. Cline. 2003. The effect of hand hygiene on illness rate among students in university residence halls. Am. J. Infect. Control 31:364– 370.